Fakhir Ansari1*
*1Senior Occupational Therapist, HOPE Rehabilitation Centre, UAE![]()
Abstract
Background and Aims: It has been established that the hazard of falling in Parkinson’s patients is a primary cause of loss of independence, and hence it is a significant component to the disease’s burden. As a result, the goal of this study is to evaluate the relationship between level of disease progression and the risk of falling in order to understand the critical need for rehab intervention in Parkinson’s disease.
Methodology: A cross-sectional survey was conducted on 24 participants participated in study through convenience sampling technique from primary and tertiary care institutes/ hospitals. Tinetti balance and gait test was used to evaluate the risk of fall among patients while The Hoehn and Yahr Scale was used to track the progression of Parkinson’s symptoms and disability.
Results: Total 24 (n=24) participants with idiopathic Parkinson’s disease were enrolled in the study in which the majority of the participants belong to age group 59-69 years. The balance and gait of Parkinson’s patient is strongly negative correlated i.e. -8.33 with the severity of Parkinson’s disease with .000 level of significance which is strongly negative correlated with the severity of Parkinson’s disease (p<0.05).
Conclusion: This demonstrates that the balance and gait of a Parkinson’s person is not related to the severity level Parkinson’s disease thus fall management in PD patients must be kept in priority during the rehab intervention for ADL independence and social participation.
Keywords: Parkinson’s disease, activities of daily livings, social participation rehabilitation, intervention, progression, fall risk management.

Introduction
Parkinson’s disease (PD) is one of the most predominant tolerant neurodegenerative illnesses that are clinically exhibited with a triad of cardinal motor and non-motor symptoms owing to neuron destruction in the substantial nigra, which produces dopamine deficiency, which compromises mobility and muscle control1. The severity of Parkinsonism in individuals increases the chance of falling. The honeymoon period of Parkinson’s disease is categorized by unilateral and moderate symptoms. As the condition progressed, it began to manifest on the contrary direction as well. The progression of the disease has an influence on possible gait and poor balance, as well as executive dysfunction, which reduces standard of living and leads to functional independence loss2,3. The gait entails muscle coordination, and the activation of coordinated muscles leads in the creation of ground response and joint forces, which are used to govern movements. Postural stability is influenced by muscle activation, kinetics, and kinematics, which impacts the tendency to fall4. Certain characteristics of a PD’s gait pattern – shortened stride length, arm swing and coordination, speed, as well as festinating and freezing of gait – are accountable for more falls when walking than any other kind of exercise5,6. Regardless of appropriate medical therapy, balance and gait problems are commonly connected with motor and non-motor aspects that improve whilst executing many activities at once. As a result, the performance of functional balance, as well as static balance, is equally significant [7][8]. The likelihood of suffering balance issues accompanied by gait freezing has yet to be investigated [9].
Falls have become more important to avoid since they are the foremost causes of impairment in Parkinson’s patients. The risk of falling is known to be increasing among the aging population, and people with Parkinson’s disease are more susceptible to falling than healthy persons of the same age10. In addition, recurring bouts of falls in PD can result in fractures at various locations, mild to serious contusions, social isolation owing to immobility, osteoporosis, and despair. It also makes patients fearful of additional falls and raises their medical costs11,12. Falls in Parkinson’s disease patients have multiple and complicated cause. In addition to core variables (age, development of the disease, gait and disparity, cognitive impairment) and secondary characteristics (anxiety, limited mobility, and weakness) that occur in reaction to a fall, an antiquity of a fall is the biggest predictor of a future fall13.
Fear of falling is a foremost risk factor for additional falls since it leads to a reduction in activity level, which leads to the injury of muscle mass and leads to balance issues [14]. The evaluation of the fall normally begins after the incident has occurred, not before. Management to lower the risk of falls should ideally begin with the prevention or postponement of falls. Even if this is not a frequent practice, it is important to assess since once the secondary features are established, effective fall management becomes a difficult task. However, the variables that cause PD patients to fall have yet to be discovered13. Clinical balance tests can be performed to assess risk factors for falls in Parkinson’s disease patients, as maintaining balance becomes difficult. It necessitates the activation of postural muscles against the gravitational pull in order to maintain posture stability. The presence of stiffness, a motor characteristic in PD, produces a disruption of the center of gravity, resulting in postural instability15. One of the most often utilized assessment tests to evaluate fall risk variables is the Tinetti Balance and Gait instrument. Tinetti’s balance scale has the advantage of incorporating both balance and gait domains with more sensitivity and reliability than other balance measures that may aid in evaluating the likelihood of falling [16]. Despite the fact that falls are the most common problem in Parkinson’s disease and can increase the risk of death and morbidity, there is no data that can help diagnose Parkinson’s disease patients who are at risk before they fall17,10.
Although some researchers have discovered that gait and balance training with motor and cognitive activities can lead to neuroplasticity, others have not8. Therapeutic activities not only enhance strength, muscular activation, and functional gait aspects, but they also reduce the risk of falling in the future. Apart from that, a comment from those who did not have the condition but participated in the fall reduction Programme was identical. As a result, fall risk prevention treatments that improve the worth of life in PD patients may lessen the impact of falls on both people and the community. To achieve this, the assessment of risk factors for falling to prioritize fall risk management in people with PD must be improved18.
It has been established that the risk of falling in Parkinson’s patients is a primary cause of loss of independence, and hence it is a significant component to the disease’s burden. The descriptive epidemiology is not well known, despite the large number of therapeutic treatments. As a result, the goal of this study is to evaluate the relationship between level of impairment and the risk of falling in order to understand the critical need for rehab intervention in Parkinson’s disease
Objectives
- To assess the gait and balance in Parkinson’s patient using Tinetti Balance and Gait Assessment Scale and relate with progression level of disability in Parkinson’s using Hoehn and Yahr scale.
- To prioritize the fall risk management for maximum participation in ADL and social activities
Methodology
Study Setting
In this study the data was collected from primary and tertiary care hospitals of Karachi including Institute of Physical Medicine and Rehabilitation (IPM&R), Liaquat National Hospital (LNH), Jinnah Postgraduate Medical Centre (JPMC) and Dr. Ziauddin Hospital (both campuses).
Target Population
Patient’s with Parkinson’s disease that holds 0.54% of prevalence in Pakistan33.
Study Design
Cross-sectional survey.
Duration of Study
Six to eight months.
Sampling Technique
Non-probability convenience sampling, clients enrolled at Ziauddin rehabilitation clinic.
Sample Selection
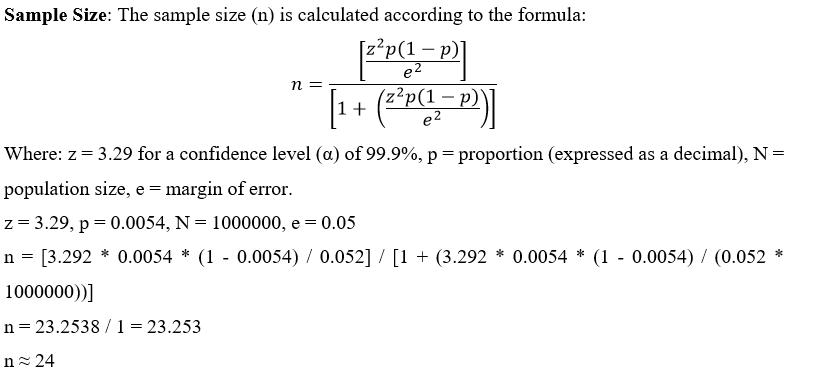
Inclusion Criteria: Both the genders diagnosed patients with Parkinson’s disease with a score of > 24 Mini-Mental State Examination (MMSE) and on stage 1 to 4 on Hoehn and Yahr Scale.
Exclusion Criteria: Bed ridden patients with secondary diagnosis or illness such as psychiatric or cardiovascular problems and those who refuse to participate.
Data Collection Tool
Tinetti balance and gait test is one of the widely used assessment scale for balance and gait to evaluate the risk of fall among Parkinson’s disease patients. It’s a three point likert scale which is comprised of two main domains. The balance scale consists of 9 items with a total of 16 points whereas the gait scale contains 8 sub-components with a sum of 12 points. The aggregate score of both components is 28. The Hoehn and Yahr Scale is used to assess the progression of Parkinson’s symptoms as well as their severity. Melvin Yahr and Margaret Hoehn first published the stages 1 to 5 in the journal Neurology in 1967. Since then, stage 0 has been introduced, as well as stages 1.5 and 2.5, which are now commonly used.
Data Collection Procedure
Respondents were enrolled in study through convenience sampling technique from primary and tertiary care hospitals.
Data Analysis
The demographic data was concluded on frequency whereas correlation was implemented to analyze the data on SPSS 22.
Results
Total 24 (n=24) participants with idiopathic Parkinson’s disease were enrolled in the study. Participants were classified into four age groups (39-49, 49-59, 59-69, 69-79 years old), in which the majority of the participants belong to age group 59-69 years. The percentage of male participants was 58.3% while the female participants were 41.7%. The economic status of most of the participants was Upper middle class with 54.2%. 20 participants were enrolled from Dr. Ziauddin Hospital while the remaining 4 participants were recruited from Sindh Institute of Physical Medicine & Rehabilitation Karachi.
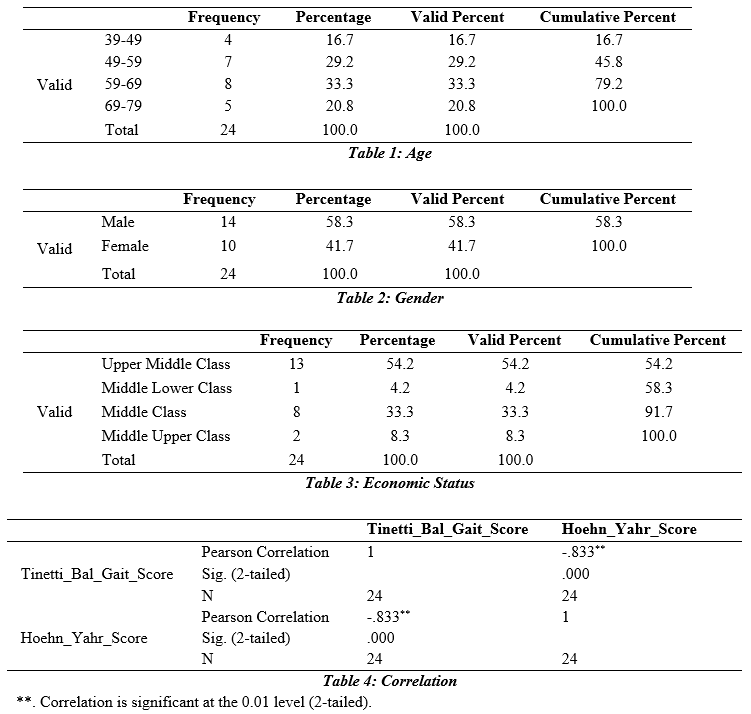
The result concluded that the balance and gait of Parkinson’s patient is strongly negative correlated i.e. -8.33 with the severity of Parkinson’s disease with .000 level of significance. This demonstrates that the balance and gait of a Parkinson’s person does not affect the severity of Parkinson’s disease.
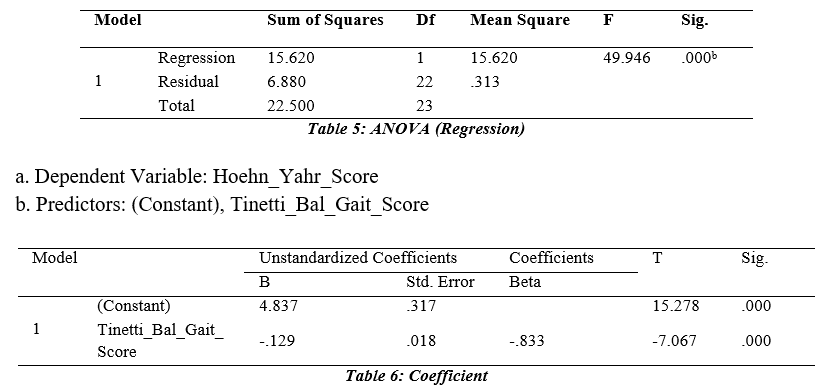
The regression model of the study would be as follows:
Y = βο + β 1 Tinetti_Bal_Gait_Score
Beta coefficients of all the variables were negative and the value of p value is less than 0.05 showing the significant positive effect. Hence, the model for the Tinetti Balance Gait Score and Hoehn and Yahr Score would be:
Hoehn and Yahr Score = 4.873 -.129Tinetti_Bal_Gait_Score The regression equation shows the value of beta coefficient of Tinetti Balance and Gait Score -.833 with the significance value 0.000, summarizing to have a significant negative effect on Hoehn and Yahr Score which shows that it has been accepted that if we increase the severity of Parkinson Disease so it will not create impairment in Balance and Gait. Besides this, the above equation shows that the Tinetti Balance and Gait Score will decrease by 12.9% if we increase Hoehn and Yahr Score by 1 unit.
Discussion
The first fall is a pivotal event lives of people living with Parkinson’s disease, frequently remembered with reasonable precision by patients and family members. This study found that the occurrence and frequency of falls, as well as the morbidity caused by them, were similar to those found in earlier studies. In summary, we discovered that falls are prevalent and repeated in patients with PD, and that they cause fractures in one-third of those who fall, as well as other serious injuries in another 25% of those who fall. Results of our study concluded that the balance and gait of Parkinson’s patient is strongly negative correlated with the severity of Parkinson’s disease (p<0.05). This demonstrates that the balance and gait of a Parkinson’s person does not correlate with the advancement of PD.
Using a number of statistical tools, the TBS and H&Y staging were determined to be the independent characteristics most connected to falls. The Tinetti test is a common quantitative evaluation that has two subscales: one for clinical balance and the other for gait analysis [19]. Similarly, in this study too the same tool is implemented to assess balance and gait in the elderly and those with Parkinson’s disease, and it’s also been shown to be a more accurate tool for predicting the risk of falling.
The majority of PD fallers in our sample were H&Y stage III or above. As a result, the shift from stage II to stage III, which coincides with the advent of postural instability, is linked to greater impairment in numerous gait-dependent tasks [20]. Balance problems, as predicted, appear to be the leading cause of falls in people with PD [21]. Individuals having PD, defects in various aspects of postural control, such as hypsometric introductory adjustments, deferred reaction time, inaccurate reflexive postural reactions, and improper axial kinesthesia, cause postural instability.
Parkinson’s disease affects impairment as the condition progresses. Gait freezing was more common among fallers, although postural instability overshadowed its statistical significance [22]. In unstable patients, gait freezing can trigger falls, and it may be the primary cause of falls in a portion of PD patients23. Nonfallers were more likely to have tremor as the primary motor symptom. These finding are steady with slower clinical development to H&Y stages III through V in parkinsonians with a tremor-dominant medical subgroup found in randomized clinical and pathological investigations24.
Previous studies have indicated that fallers had lower MMSE scores25,26. Although it was not specifically measured in our investigation, however there might be a relationship between cognitive function and gait and postural problems. In PD sufferers, however, cognitive decline might be an epiphenomenon linked to more severe illness. Regardless of the lack of blood pressure measurements in the supine and standing postures, our study showed no statistically significant differences in the history of orthostatic hypotension symptoms between fallers and non-fallers. The fact that the risk of falling with Parkinson’s disease increased markedly with age, especially after the age of 70, was an interesting discovery. As a result, ageing appears to play a critical role in advanced PD, probably by speeding up the underlying disease process, allowing neuropath logical abnormalities in brain areas related to gait and balance control to multiply rapidly in the late stages of the illness. Furthermore, those who exhibited clinical symptoms of PD beyond the age of 70 had falls considerably sooner than those who had symptoms earlier in life, demonstrating the combined effect of ageing and disease development. The retrospective evaluation of falls is one of the study’s limitations. It has been argued that the elderly has a proclivity for forgetting past falls. The precision of dating past falls, on the other hand, may be influenced by how people are interrogated. With the help of patients, family, careers, and clinical records, we were able to date the falls, mainly the primary one 27
Even though the Tinetti test and H&Y stages have minimal prognosis for falls in Parkinson’s disease, and more advanced tests of balance impairments in PD should be produced, these simple and rapid clinical assessments may help to identify high-risk people. Exercise regimens focusing
on balance have been shown to be particularly beneficial in avoiding falls in older adults without Parkinson’s disease. Targeted exercise improves balance in people with Parkinson’s disease, and cueing training can help with gait freezing28,29,30. Seeking new fall predictors in PD patients — possibly utilizing post urography or other electrophysiological gadgets for measuring postural stability—as well as creating treatment techniques to enhance balance and avoid falls in these patients should be the focus of future research31,32.
AUTHORS’ CONTRIBUTION:
The following authors have made substantial contributions to the manuscript as under:
Conception or Design: Fakhir Ansari
Acquisition, Analysis or Interpretation of Data: Fakhir Ansari
Manuscript Writing & Approval: Fakhir Ansari
All authors acknowledge their accountability for all facets of the research, ensuring that any concerns regarding the accuracy or integrity of the work are duly investigated and resolved.
ACKNOWLEDGEMENTS: We thanks all the participants in this study.
INFORMED CONSENT: Written Informed Consent was taken from each patient.
CONFLICT OF INTEREST: The author (s) have no conflict of interest regarding any of the activity perform by PJR.
FUNDING STATEMENTS: None declared
ETHICS STATEMENTS: N/A
References
- Hughes, R.C., 1994. Parkinson’s Disease and its Management. BMJ: British Medical Journal, 308(6923), p.281.
- Lindholm, B., Hagell, P., Hansson, O. And Nilsson, M.H., 2015. Prediction of falls and/or near falls in people with mild Parkinson’s disease. Plos one, 10(1), p.e0117018.
- Sveinbjornsdottir, S., 2016. The clinical symptoms of Parkinson’s disease. Journal of neurochemistry, 139, pp.318-324.
- Creaby, M.W. and Cole, M.H., 2018. Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism & related disorders, 57, pp.1-8.
- Kim, S.M., Kim, D.H., Yang, Y., Ha, S.W. and Han, J.H., 2018. Gait patterns in Parkinson’s disease with or without cognitive impairment. Dementia and neurocognitive disorders, 17(2), p.57.
- Curtze, C., Nutt, J.G., Carlson-Kuhta, P., Mancini, M. And Horak, F.B., 2016. Objective gait and balance impairments relate to balance confidence and perceived mobility in people with Parkinson disease. Physical therapy, 96(11), pp.1734-1743.
- Del Din, S., Galna, B., Godfrey, A., Bekkers, E.M., Pelosin, E., Nieuwhof, F., Mirelman, A., Hausdorff, J.M. and Rochester, L., 2019. Analysis of free-living gait in older adults with and without Parkinson’s disease and with and without a history of falls: identifying generic and disease-specific characteristics. The Journals of Gerontology: Series A, 74(4), pp.500-506.
- Conradsson, D., Löfgren, N., Nero, H., Hagströmer, M., Ståhle, A., Lökk, J. And Franzén, E., 2015. The effects of highly challenging balance training in elderly with Parkinson’s disease: a randomized controlled trial. Neurorehabilitation and neural repair, 29(9), pp.827-836.
- Gao, C., Liu, J., Tan, Y. And Chen, S., 2020. Freezing of gait in Parkinson’s disease: pathophysiology, risk factors and treatments. Translational neurodegeneration, 9, pp.1-22.
- Farombi, T.H., Owolabi, M.O. and Ogunniyi, A., 2016. Falls and their associated risks in Parkinson’s disease patients in Nigeria. Journal of movement disorders, 9(3), p.160.
- Tufail, M., 2019. Clinical features and risk factors of Parkinson’s disease in a population of Khyber Pakhtunkhwa, Pakistan: a case-control study. Neurodegenerative Diseases, 19(5-6), pp.211-217.
- Pelicioni, P.H., Menant, J.C., Latt, M.D. and Lord, S.R., 2019. Falls in Parkinson’s disease subtypes: risk factors, locations and circumstances. International journal of environmental research and public health, 16(12), p.2216.
- Lord, S., Galna, B., Yarnall, A.J., Morris, R., Coleman, S., Burn, D. And Rochester, L., 2017. Natural history of falls in an incident cohort of Parkinson’s disease: early evolution, risk and protective features. Journal of neurology, 264(11), pp.2268-2276.
- Saad, S., Nomani, A.Z., Badshah, M. And Afzal, A., 2017. Frequency of non-motor symptoms in Parkinson disease: experience from Pakistan. Pakistan Journal of Neurological Sciences (PJNS), 12(1), pp.8-15.
- Park, J.H., Kang, Y.J. and Horak, F.B., 2015. What is wrong with balance in Parkinson’s disease?. Journal of movement disorders, 8(3), p.109.
- Parrington, L., Popa, B., Martini, D.N., Chesnutt, J.C. and King, L.A., 2020. Instrumented balance assessment in mild traumatic brain injury: Normative values and descriptive data for acute, sub-acute and chronic populations. Journal of Concussion, 4, p.2059700220975605.
- Abbas, S., Munawar, A. And Al Mostafa, M.Y., 2021. Prevalence of Balance Impairments in Individuals with Parkinson Disease. Pakistan Journal of Physical Therapy (PJPT), pp.26-31.
- Shaikh, H.A. and Masood, S., 2019. Effects of physiotherapy interventions on fall, posture and quality of life in parkinson disease. Pakistan journal of rehabilitation, 8(1), pp.04-12.
- Syed, N.A., Ali, F., Sher, K., Ikram, A., Soomro, B., Shahbaz, N., Sheerani, M., Jamil, S., Numan, A., Lakhair, M. And Awan, I., 2015. National guidelines for diagnosis and management of Parkinson’s disease in Pakistan. Pakistan Journal of Neurological Sciences (PJNS), 10(1), pp.40-48.
- Farooqui, S.I., 2017. ROLE OF LEE SILVERMAN VOICE TREATMENT (LSVT) IN PARKINSON DISEASE. Pakistan Journal of Rehabilitation, 6(2), pp.1-2.
- Fanning, S., Selkoe, D. And Dettmer, U., 2020. Parkinson’s disease: proteinopathy or lipidopathy?. NPJ Parkinson’s disease, 6(1), pp.1-9.
- Hayes, M.T., 2019. Parkinson’s disease and parkinsonism. The American journal of medicine, 132(7), pp.802-807.
- Rajput, A.H., Voll, A., Rajput, M.L., Robinson, C.A. and Rajput, A., 2009. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology, 73(3), pp.206-212.
- Kempster, P.A., O’Sullivan, S.S., Holton, J.L., Revesz, T. And Lees, A.J., 2010. Relationships between age and late progression of Parkinson’s disease: a clinicopathological study. Brain, 133(6), pp.1755-1762.
- Jankovic, J. And Tan, E.K., 2020. Parkinson’s disease: Etiopathogenesis and treatment. Journal of Neurology, Neurosurgery & Psychiatry, 91(8), pp.795-808.
- Holmes, J.D., Jenkins, M.E., Johnson, A.M., Adams, S.G. and Spaulding, S.J., 2010. Dual-task interference: the effects of verbal cognitive tasks on upright postural stability in Parkinson’s disease. Parkinson’s Disease, 2010
- Romano, S., Savva, G.M., Bedarf, J.R., Charles, I.G., Hildebrand, F. And Narbad, A., 2021. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. Npj Parkinson’s Disease, 7(1), pp.1-13.
- Sherrington, C., Whitney, J.C., Lord, S.R., Herbert, R.D., Cumming, R.G. and Close, J.C., 2008. Effective exercise for the prevention of falls: a systematic review and meta‐ Journal of the American Geriatrics Society, 56(12), pp.2234-2243
- Fearon, C. And Fasano, A., 2021. Parkinson’s disease and the COVID-19 pandemic. Journal of Parkinson’s Disease, 11(2), pp.431-444.
- Chung, K.A., Lobb, B.M., Nutt, J.G. and Horak, F.B., 2010. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology, 75(14), pp.1263-1269
- Adkin, A.L., Bloem, B.R. and Allum, J.H.J., 2005. Trunk sway measurements during stance and gait tasks in Parkinson’s disease. Gait & posture, 22(3), pp.240-249.
- Ganesan, M., Pal, P.K., Gupta, A. And Sathyaprabha, T.N., 2010. Dynamic posturography in evaluation of balance in patients of Parkinson’s disease with normal pull test: concept of a diagonal pull test. Parkinsonism & related disorders, 16(9), pp.595-599.
- Tufail M. Clinical features and risk factors of Parkinson’s disease in a population of Khyber Pakhtunkhwa, Pakistan: a case-control study. Neurodegenerative Diseases. 2019;19(5-6):211-7.
The Ziauddin University is on the list of I4OA, I4OC, and JISC.
This is an open- access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
